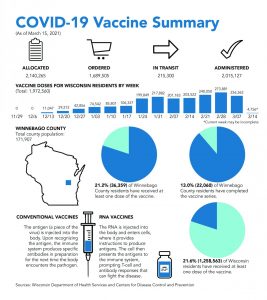
COVID-19 vaccine history
March 17, 2021
In 1796, English physician Edward Jenner first demonstrated the concept of a vaccine when he inoculated a 13-year-old boy with the vaccinia virus, or cowpox. The young boy eventually became immune to smallpox, and in 1798 the smallpox vaccine was officially developed. Smallpox was globally eradicated in 1979.
Jenner’s discovery of immunization for a virus with a devastating death toll led to thousands of scientists experimenting with other deadly viruses and bacteria to create more vaccines throughout the next few centuries.
According to the Centers for Disease Control and Prevention, immunization is important for protection against viruses and certain bacterial infections. Traditional vaccines provide an opportunity for the body to be exposed to an inactivated form of a foreign agent that causes an immune reaction.
“A vaccine stimulates your immune system to produce antibodies, exactly like it would if you were exposed to the disease. After getting vaccinated, you develop immunity to that disease, without having to get the disease first,” the CDC website said.
The body builds up immunity through the development of antibodies that will later be used to fight against the same disease if the body is exposed to it again.
The COVID-19 vaccine, however, is different compared to traditional vaccines. This vaccine uses mRNA, which is a molecule derived from RNA that contains information needed to build proteins for the cell.
Specifically, the vaccine contains mRNA from SARS-CoV-2 that provides instructions for building a spike protein.
“Once displayed on the cell surface, the protein or antigen causes the immune system to begin producing antibodies and activating T-cells to fight off what it thinks is an infection,” the CDC website said.
As for the production of traditional vaccines, the process can take anywhere from 10 to 15 years due to the amount of research and regulation required for it to be approved for public use.
Vaccines typically require several years of research and development, three phases of trials with human participants and final approval by the CDC and FDA.
The Pfizer, Moderna and Johnson & Johnson vaccine production and approval took less than one year. Multiple factors contributed to this quick timeline.
Scientists have been investigating the potential use of mRNA in vaccines for the past several decades. mRNA vaccines have been tested for the flu, Zika, rabies and cytomegalovirus, but none have been approved for the public. With the readily available knowledge of past experiments, vaccine development was made easier for COVID-19.
The biological mechanisms of mRNA technology itself is another reason why the vaccine was developed and approved quickly.
“mRNA vaccines have several benefits compared to other types of vaccines including use of a non-infectious element, shorter manufacturing times and potential for targeting of multiple diseases,” the CDC website said. “mRNA vaccines can be developed in a laboratory using a DNA template and readily available materials.”
Other reasons for the fast approval of the three vaccines include substantial funding from the U.S. government and private donors. Also, the FDA implemented Emergency Use Authorization on Dec. 11, 2020, for Pfizer and Moderna vaccines and on Feb. 27, 2021, for the Johnson & Johnson vaccine.













